Chemical Engineering Gate Yearwise
Chemical Eng. Gate 2024
Chemical Eng. Gate 2023
Chemical Eng. Gate 2022
Chemical Eng. Gate 2021
Chemical Eng. Gate 2020
Chemical Eng. Gate 2019
Chemical Eng. Gate 2018
Chemical Eng. Gate 2017
Chemical Eng. Gate 2016
Chemical Eng. Gate 2015
Chemical Eng. Gate 2014
Chemical Eng. Gate 2013
Chemical Eng. Gate 2012
Chemical Eng. Gate 2011
Chemical Eng. Gate 2010
Chemical Engineering Gate 2022 Questions with Answer
Ques 53 Gate 2022
The value of (1+𝑖)12 , where 𝑖=√−1, is
Ques 54 Gate 2022
Let 𝑓(𝑥)= 𝑒-|x|, where 𝑥 is real. The value of df/dx at 𝑥=−1 is
Ques 55 Gate 2022
The value of the real variable 𝑥≥0, which maximizes the function f(𝑥)= 𝑥𝑒e-x is
Ques 56 GATE 2022
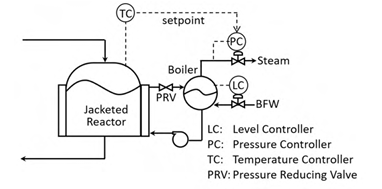
A control system on the jacket side of a reactor is shown in the figure. Pressurized water flows through the jacket to cool the reactor. The heated water flashes in the boiler. The exothermic reaction heat thus generates steam. Fresh boiler feed water (BFW) is added to make-up for the loss of water as steam. Assume that all control valves are air-to-open. The controller action, ‘direct’ or ‘reverse’, is defined with respect to the controller. Select the option that correctly specifies the action of the controllers.
Ques 57 GATE 2022
Liquid flowing through a heat exchanger (HX) is heated. A bypass stream is provided to control the temperature of the heated exit stream. From the given plumbing options, the one that provides the most effective temperature control for large disturbances while avoiding vaporization in the heat exchanger is
Ques 58 GATE 2022
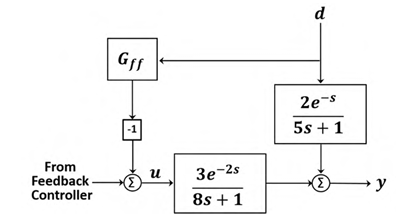
The appropriate feedforward compensator, 𝐺ff , in the shown block diagram is
Ques 59 GATE 2022
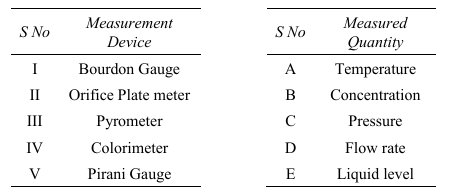
Choose the option that correctly pairs the given measurement devices with the quantities they measure.
Ques 60 GATE 2022
For a single component system at vapor-liquid equilibrium, the extensive variables 𝐴, 𝑉, 𝑆 and 𝑁 denote the Helmholtz free energy, volume, entropy, and number of moles, respectively, in a given phase. If superscripts (𝑣) and (𝑙) denote the vapor and liquid phase, respectively, the relation that is NOT CORRECT is
Ques 61 GATE 2022
5 moles of liquid benzene, 8 moles of liquid toluene and 7 moles of liquid xylene are mixed at 25 °C and 1 bar. Assuming the formation of an ideal solution and using the universal gas constant 𝑅 = 8.314 J mol-1 K-1, the total entropy change is __________ J K-1 (rounded off to one decimal place).
Ques 62 GATE 2022
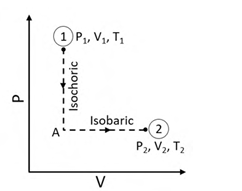
N moles of an ideal gas undergo a two-step process as shown in the figure. Let P, V, and T denote the pressure, volume, and temperature of the gas, respectively. The gas, initially at state-1 (P1, V1, T1), undergoes an isochoric (constant volume) process to reach state-A, and then undergoes an isobaric (constant pressure) expansion to reach state-2 (P2, V2, T2). For an ideal gas, CP – CV = NR, where CP and CV are the heat capacities at constant pressure and constant volume, respectively, and assumed to be temperature independent. The heat gained by the gas in the two-step process is given by
Ques 63 GATE 2022
Consider the process in the figure. The liquid phase elementary reactions
A + B → P −r = k xA xB
A + B → S −r = k xA xB
B + A → 2P −r = k xB xA
occur in the continuous stirred tank reactor (CSTR), where x is the mole fraction of the jth component (j = A, B, P, S) in the CSTR. It is given that k = k1. All process feed, process exit and recycle streams are pure. At steady state, the net generation rate of the undesired product, S, in the CSTR is zero. As q = xA / xB is varied at constant reactor temperature, the reactor volume is adjusted to maintain a constant single-pass conversion of B. For a fixed product rate and 90% conversion of B in the reactor, the value of q that minimizes the sum of the molar flow rates of the A and S recycle streams is ________ (round off to one decimal place).
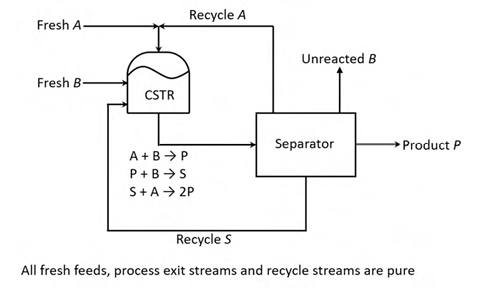
Ques 64 GATE 2022
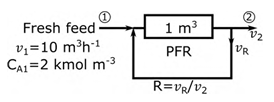
An elementary irreversible liquid-phase reaction, 2A → B, is carried out under isothermal conditions in a 1 m3 ideal plug flow reactor (PFR) as shown in the figure. The volumetric flow rate of fresh A, v = 10 m3 h−1, and its concentration CA = 2 kmol m−3. For a recycle ratio R = 0, the conversion of A at location 2 with respect to the fresh feed (location 1) is 50%. For R → ∞, the corresponding conversion of A is _________% (rounded off to one decimal place).
Ques 65 GATE 2022
An elementary irreversible gas-phase reaction, A → B + C, is carried out at fixed temperature and pressure in two separate ideal reactors: (i) a 10 m3 plug flow reactor (PFR), (ii) a 10 m3 continuous-stirred tank reactor (CSTR). If pure A is fed at 5 m3 h−1 to the PFR operating at 400 K, the conversion is 80%. If a mixture of 50 mol% of A and 50 mol% of an inert is fed at 5 m3 h−1 to the CSTR operating at 425 K, the conversion is 80%. The universal gas constant R = 8.314 J mol−1 K−1. Assuming the Arrhenius rate law, the estimated activation energy is __________ kJ mol−1 (rounded off to one decimal place).

Total Unique Visitors