Chemical Engineering Gate Yearwise
Chemical Eng. Gate 2024
Chemical Eng. Gate 2023
Chemical Eng. Gate 2022
Chemical Eng. Gate 2021
Chemical Eng. Gate 2020
Chemical Eng. Gate 2019
Chemical Eng. Gate 2018
Chemical Eng. Gate 2017
Chemical Eng. Gate 2016
Chemical Eng. Gate 2015
Chemical Eng. Gate 2014
Chemical Eng. Gate 2013
Chemical Eng. Gate 2012
Chemical Eng. Gate 2011
Chemical Eng. Gate 2010
Chemical Engineering Gate 2023 Questions with Answer
Ques 1 GATE 2023
Which one of the following statements related to octane number is NOT correct?
Ques 2 GATE 2023
Which one of the following options represents the major components of oleum?
Ques 3 GATE 2023
For a reversible endothermic chemical reaction with constant heat of reaction over the operating temperature range, 𝐾 is the thermodynamic equilibrium constant. Which one of the following figures shows the CORRECT dependence of 𝐾 on temperature 𝑇?
Ques 4 GATE 2023
Nitrile rubber is manufactured via polymerization process. Which one of the following options is the CORRECT pair of monomers used in this process?
Ques 5 GATE 2023
Spray dryers have many advantages. Which one of the following is NOT an advantage of a typical spray dryer?
Ques 6 GATE 2023
A liquid surge tank has Fin and Fout as the inlet and outlet flow rates respectively, as shown in the figure below. Fout is proportional to the square root of the liquid level h. The cross-sectional area of the tank is 20 cm2. Density of the liquid is constant everywhere in the system. At steady state, Fin = Fout = 10 cm3s−1 and h = 16 cm. The variation of h with Fin is approximated as a first order transfer function. Which one of the following is the CORRECT value of the time constant (in seconds) of this system?
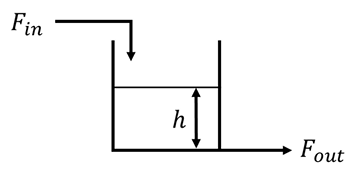
Ques 7 GATE 2023
An isothermal jacketed continuous stirred tank reactor (CSTR) operating at 150 °C is shown in the figure below. The cold feed entering the system at 30 °C is preheated to a temperature T (T < 150 °C) using a heat exchanger HX1. This preheated feed is further heated to 150 °C using the utility heater HX2. The mass flow rate and heat capacity are same for all the process streams, and the overall heat transfer coefficient is independent of temperature. Which one of the following statements is the CORRECT action to take if it is desired to increase the value of T?
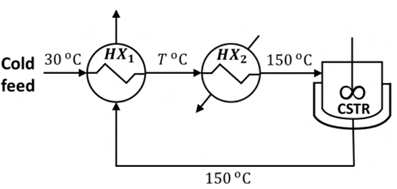
Ques 8 GATE 2023
Burning of methane in a combustor yields carbon monoxide, carbon dioxide, and water vapor. Methane is fed to the combustor at 100 mol.hr−1, of which 50% reacts. The theoretical oxygen requirement (in mol.hr−1) is ________ (rounded off to one decimal place).
Ques 9 GATE 2023
Match the products in Group 1 with the manufacturing processes in Group 2 listed in the table below.
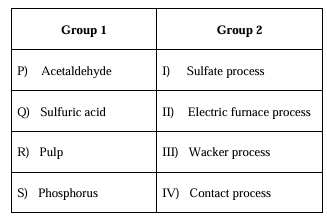
Ques 10 GATE 2023
Match the reactions in Group 1 with the catalysts in Group 2 listed in the table below.
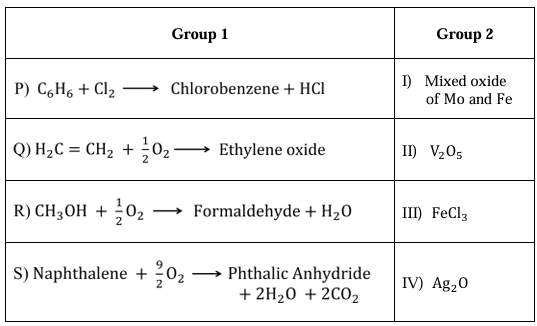
Ques 11 GATE 2023
Water in a container at 290 K is exposed to air containing 3% CO2 by volume. Air behaves like an ideal gas and is maintained at 100 kPa pressure. The liquid phase comprising of dissolved CO2 in water behaves like an ideal solution. Use Henry’s constant of CO2 dissolved in water at 290 K as 12 MPa. Under equilibrium conditions, which one of the following is the CORRECT value of the mole fraction of CO2 dissolved in water?
Ques 12 GATE 2023
The enthalpy (H, in J·mol−1) of a binary liquid system at constant temperature and pressure is given as H = 40x1 + 60x2 + x1x2(4x1 + 2x2), where x1 and x2 represent the mole fractions of species 1 and 2 in the liquid, respectively. Which one of the following is the CORRECT value of the partial molar enthalpy of species 1 at infinite dilution, H1∞ (in J·mol−1)?
Ques 13 GATE 2023
CO and H2 participate in a catalytic reaction. The partial pressures (in atm) of the reacting species CO and H2 in the feed stream are pCO and pH₂, respectively. While CO undergoes molecular adsorption, H2 adsorbs via dissociative adsorption, that is, as hydrogen atoms. The equilibrium constants (in atm−1) corresponding to adsorption of CO and H2 to the catalyst sites are KCO and KH₂, respectively. Total molar concentration of active sites per unit mass of the catalyst is Ct (in mol·(g cat)−1). Both the adsorption steps are at equilibrium. Which one of the following expressions is the CORRECT ratio of the concentration of catalyst sites occupied by CO to that by hydrogen atoms?

Total Unique Visitors