Chemical Engineering Gate Yearwise
Chemical Eng. Gate 2024
Chemical Eng. Gate 2023
Chemical Eng. Gate 2022
Chemical Eng. Gate 2021
Chemical Eng. Gate 2020
Chemical Eng. Gate 2019
Chemical Eng. Gate 2018
Chemical Eng. Gate 2017
Chemical Eng. Gate 2016
Chemical Eng. Gate 2015
Chemical Eng. Gate 2014
Chemical Eng. Gate 2013
Chemical Eng. Gate 2012
Chemical Eng. Gate 2011
Chemical Eng. Gate 2010
Chemical Engineering Gate 2019 Questions with Answer
Ques 53 GATE 2019
A fractionator recovers 95 mol % n-propane as the distillate from an equimolar mixture of n-propane and n-butane. The condensate is a saturated liquid at 55 °C. The Antoine equation is of the form, ln(psat [in bar]) = A - B/(T[in K] + C), and the constants are provided below:
n-propane: A=9.1058, B=1872.46, C=-25.16
n-butane: A=9.0580, B=2154.90, C=-34.42
Assuming Raoult''s law, the condenser pressure (in bar) is ______ (rounded off to one decimal place).'
Ques 54 GATE 2019
Two spherical camphor particles of radii 20 cm and 5 cm, far away from each other, are undergoing sublimation in a stream of air. The mass transfer coefficient is proportional to 1/√r(t), where r(t) is the radius of the sphere at time t. Assume that the partial pressure of camphor far away from the surface of the particle is zero. Also, assume quasi-steady state, identical ambient conditions, and negligible heat effects. If t1 and t2 are the times required for complete sublimation of the 20 cm and 5 cm camphor particles, respectively, the ratio t1/t2 is ______ (rounded off to one decimal place).
Ques 55 GATE 2019
A countercurrent absorption tower is designed to remove 95% of component A from an incoming binary gas mixture using pure solvent B. The mole ratio of A in the inlet gas is 0.02. The carrier gas flow rate is 50 kmol/h. The equilibrium relation is given by Y = 2X, where Y and X are the mole ratios of A in the gas and liquid phases, respectively. If the tower is operated at twice the minimum solvent flow rate, the mole ratio of A in the exit liquid stream is ______ (rounded off to three decimal places).
Ques 56 GATE 2019
A binary mixture with components A and B is to be separated in a distillation column to obtain 95 mol% A as the top product. The binary mixture has a constant relative volatility αAB = 2. The column feed is a saturated liquid containing 50 mol% A. Under the usual simplifying assumptions such as constant molal overflow, negligible heat loss, ideal trays, the minimum reflux ratio for this separation is ______ (rounded off to one decimal place).
Ques 57 GATE 2019
disk turbine is used to stir a liquid in a baffled tank. To design the agitator, experiments are performed in a lab-scale model with a turbine diameter of 0.05 m and a turbine impeller speed of 600 rpm. The liquid viscosity is 0.001 Pa s while the liquid density is 1000 kg/m3. The actual application has a turbine diameter of 0.5 m, an impeller speed of 600 rpm, a liquid viscosity of 0.1 Pa s and a liquid density of 1000 kg/m3. The effect of gravity is negligible. If the power required in the lab-scale model is P1 and the estimated power for the actual application is P2, then the ratio P2/P1 is
Ques 58 GATE 2019
Consider two competing equipment A and B. For a compound interest rate of 10% per annum, in order for equipment B to be the economically cheaper option, its minimum life (in years) is ______ (rounded off to the next higher integer).

Ques 59 GATE 2019
A taxi-car is bought for Rs 10 lakhs. Its salvage value is zero. The expected yearly income after paying all expenses and applicable taxes is Rs 3 lakhs. The compound interest rate is 9% per annum. The discounted payback period (in years), is ______ (rounded off to the next higher integer).
Ques 60 GATE 2019
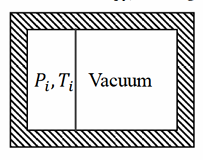
One mole of an ideal gas is contained in one part of a rigid, perfectly insulated container partitioned by a thin membrane; the other part is evacuated.
The initial pressure and temperature of the gas are Pi and Ti respectively.
The membrane ruptures and the gas expands to fill the entire volume; the final equilibrium pressure is Pf = Pi/4.
Given molar heat capacities Cp, Cv and the gas constant R (all in the same units as molar entropy), find the change in molar entropy Sf − Si.
Ques 61 GATE 2019
For a fully-developed turbulent hydrodynamic boundary layer for flow past a flat plate, the thickness of the boundary layer increases with distance x from the leading edge of the plate, along the free-stream flow direction, as
Ques 62 GATE 2019
For a binary nonideal A-B mixture exhibiting a minimum boiling azeotrope, the activity coefficients, γ i (i=A,B), must satisfy
Ques 63 GATE 2019
Carbon monoxide (CO) reacts with hydrogen sulphide (H2S) at a constant temperature of 800 K and a constant pressure of 2 bar as:
CO + H2S ⇌ COS + H2
The Gibbs free energy of the reaction Δg°rxn = 22972.3 J/mol and universal gas constant R=8.314 J/(mol K). Both the reactants and products can be assumed to be ideal gases. If initially only 4 mol of H2S and 1 mol of CO are present, the extent of the reaction (in mol) at equilibrium is ______ (rounded off to two decimal places).
Ques 64 GATE 2019
For a given binary system at constant temperature and pressure, the molar volume (in m3/mol) is given by: v = 30xA + 20xB + xAxB (15xA - 7xB), where xA and xB are the mole fractions of components A and B, respectively. The volume change of mixing Δvmix (in m3/mol) at xA = 0.5 is ______ (rounded off to one decimal place).
Ques 65 GATE 2019
Consider a vessel containing steam at 180 °C. The initial steam quality is 0.5 and the initial volume of the vessel is 1 m3. The vessel loses heat at a constant rate q̇ under isobaric conditions so that the quality of steam reduces to 0.1 after 10 hours. The thermodynamic properties of water at 180 °C are (subscript g: vapor phase; subscript f: liquid phase): specific volume: vg=0.19405 m3/kg, vf=0.001127 m3/kg; specific internal energy: ug=2583.7 kJ/kg, uf=762.08 kJ/kg; specific enthalpy: hg=2778.2 kJ/kg, hf=763.21 kJ/kg. The rate of heat loss q̇ (in kJ/hour) is ______ (rounded off to the nearest integer).

Total Unique Visitors