Chemical Engineering Gate Yearwise
Chemical Eng. Gate 2024
Chemical Eng. Gate 2023
Chemical Eng. Gate 2022
Chemical Eng. Gate 2021
Chemical Eng. Gate 2020
Chemical Eng. Gate 2019
Chemical Eng. Gate 2018
Chemical Eng. Gate 2017
Chemical Eng. Gate 2016
Chemical Eng. Gate 2015
Chemical Eng. Gate 2014
Chemical Eng. Gate 2013
Chemical Eng. Gate 2012
Chemical Eng. Gate 2011
Chemical Eng. Gate 2010
Chemical Engineering Gate 2017 Questions with Answer
Ques 53 GATE 2017
Match the polymerization processes in Group-1 with the polymers in Group-2.

Ques 54 GATE 2017
An aqueous salt-solution enters a crystallizer operating at steady state at 25°C. The feed temperature is 90°C and the salt concentration in the feed is 40 weight %. The salt crystallizes as a pentahydrate. The crystals and the mother liquor leave the crystallizer. The molecular weight of the anhydrous salt is 135. The solubility of the salt at 25°C is 20 weight %. The feed flowrate required for a production rate of 100 kg/s of the hydrated salt, rounded to the nearest integer, is
Ques 55 GATE 2017
The reversible reaction of t-butyl alcohol (TBA) and ethanol (EtOH) to ethyl t-butyl ether (ETBE) is
TBA + EtOH ⇌ ETBE + Water
The equilibrium constant for this reaction is Kc = 1. Initially, 74 g of TBA is mixed with 100 g of aqueous solution containing 46 weight% ethanol. The molecular weights are: 74 g/mol for TBA, 46 g/mol for EtOH, 102 g/mol for ETBE, and 18 g/mol for water. The mass of ETBE at equilibrium, rounded to 1 decimal place, is
Ques 56 GATE 2017
Match the variables in Group-1 with the instruments in Group-2. Group-1 P) Temperature Q) Liquid level R) Vacuum S) Concentration Group-2 I) Capacitance probe II) McLeod gauge III) Chromatograph IV) Thermistor Choose the correct set of combinations.
Ques 57 GATE 2017
An LVDT (Linear Variable Differential Transformer) is a transducer used for converting
Ques 58 GATE 2017
The transfer function of a system is 1 / (4s2 + 1.2s + 1) For a unit step increase in the input, the fractional overshoot, rounded to 2 decimal places, is
Ques 59 GATE 2017
The open loop transfer function of a process with a proportional controller (gain Kc) is GOL = Kc e-2s / s Based on the Bode criterion for closed-loop stability, the ultimate gain of the controller, rounded to 2 decimal places, is
Ques 60 GATE 2017
The characteristic equation of a closed-loop system is 6s3 + 11s2 + 6s + (1+K) = 0, where K > 0 The value of K beyond which the system just becomes unstable, rounded to the nearest integer, is
Ques 61 GATE 2017
The volumetric properties of two gases M and N are described by the generalized compressibility chart which expresses the compressibility factor (Z) as a function of reduced pressure and reduced temperature only. The operating pressure (P) and temperature (T) of two gases M and N along with their critical properties (Pc, Tc) are given in the table below.
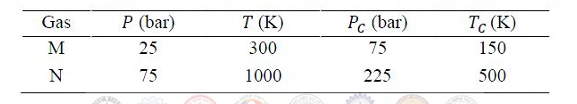
Ques 62 GATE 2017
Water is heated at atmospheric pressure from 40°C to 80°C using two different processes. In process I, the heating is done by a source at 80°C. In process II, the water is first heated from 40°C to 60°C by a source at 60°C, and then from 60°C to 80°C by another source at 80°C. Identify the correct statement.
Ques 63 GATE 2017
The pressure of a liquid is increased isothermally. The molar volume of the liquid decreases from 50.45×10-6 m3/mol to 48×10-6 m3/mol during this process. The isothermal compressibility of the liquid is 10-9 Pa-1, which can be assumed to be independent of pressure. The change in the molar Gibbs free energy of the liquid, rounded to nearest integer, is
Ques 64 GATE 2017
A sparingly soluble gas (solute) is in equilibrium with a solvent at 10 bar. The mole fraction of the solvent in the gas phase is 0.01. At the operating temperature and pressure, the fugacity coefficient of the solute in the gas phase and the Henry's law constant are 0.92 and 1000 bar, respectively. Assume that the liquid phase obeys Henry's law. The MOLE PERCENTAGE of the solute in the liquid phase, rounded to 2 decimal places, is
Ques 65 GATE 2017
The vapour pressure of a pure substance at a temperature T is 30 bar. The actual and ideal gas values of g/RT for the saturated vapour at this temperature T and 30 bar are 7.0 and 7.7, respectively. Here, g is the molar Gibbs free energy and R is the universal gas constant. The fugacity of the saturated liquid at these conditions, rounded to 1 decimal place, is

Total Unique Visitors