Chemical Engineering Gate Yearwise
Chemical Eng. Gate 2024
Chemical Eng. Gate 2023
Chemical Eng. Gate 2022
Chemical Eng. Gate 2021
Chemical Eng. Gate 2020
Chemical Eng. Gate 2019
Chemical Eng. Gate 2018
Chemical Eng. Gate 2017
Chemical Eng. Gate 2016
Chemical Eng. Gate 2015
Chemical Eng. Gate 2014
Chemical Eng. Gate 2013
Chemical Eng. Gate 2012
Chemical Eng. Gate 2011
Chemical Eng. Gate 2010
Chemical Engineering Gate 2015 Questions with Answer
Ques 53 GATE 2015
A multi-stage, counter-current liquid-liquid extractor is used to separate solute C from a binary mixture (F) of A and C using solvent B. Pure solvent B is recovered from the raffinate R by distillation, as shown in the schematic diagram below. Locations of different mixtures for this process are indicated on the triangular diagram below.
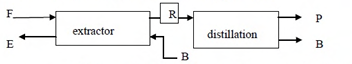
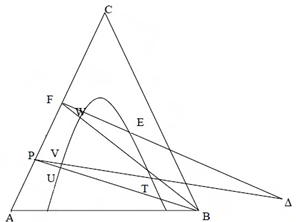
Ques 54 GATE 2015
A binary feed consisting of 25 mol% liquid and 75 mol% vapour is separated in a staged distillation column. The mole fraction of the more volatile component in the distillate product is 0.95. The molar flow rate of distillate is 50% of the feed flow rate and the McCabe-Thiele method can be used to analyze the column. The q-line intersects the operating line of the enriching section at (0.35, 0.5) on the x-y diagram. The slope of the stripping section operating line (up to one decimal place) is _______.
Ques 55 GATE 2015
The diameters of sand particles in a sample range from 50 to 150 microns. The number of particles of diameter x in the sample is proportional to 1/(50 + x). The average diameter, in microns, (up to one decimal place) is _______
Ques 56 GATE 2015
A typical batch filtration cycle consists of filtration followed by washing. One such filtration unit operating at constant pressure difference first filters a slurry during which 5 liters of filtrate is collected in 100 s. This is followed by washing, which is done for tw seconds and uses 1 liter of wash water. Assume the following relation to be applicable between the applied pressure drop ΔP, cake thickness L at time t, and volume of liquid V collected in time t: ΔP/L = k1 dV/dt ; L = k2 V, if L is changing. k1 and k2 can be taken to be constant during filtration and washing. The wash time tw, in seconds (up to one decimal place), is _______.
Ques 57 GATE 2015
The cost of two independent process variables f1 and f2 affects the total cost CT (in lakhs of rupees) of the process as per the following function: CT = 1000/(f1f2) + 20f12 + 50f22 + 50 The lowest total cost CT, in lakhs of rupees (up to one decimal place), is _______.
Ques 58 GATE 2015
A proposed chemical plant is estimated to have a fixed capital (FC) of Rs. 24 crores. Assuming other costs to be small, the total investment may be taken to be same as FC. After commissioning (at t = 0 years), the annual profit before tax is Rs. 10 crores /year (at the end of each year) and the expected life of the plant is 10 years. The tax rate is 40% per year and a linear depreciation is allowed at 10% per year. The salvage value is zero. If the annual interest rate is 12%, the NPV (net present value or worth) of the project in crores of rupees (up to one decimal place) is _______.
Ques 59 GATE 2015
For a pure liquid, the rate of change of vapour pressure with temperature is 0.1 bar/K in the temperature range of 300 to 350 K. If the boiling point of the liquid at 2 bar is 320 K, the temperature (in K) at which it will boil at 1 bar (up to one decimal place) is _______.
Ques 60 GATE 2015
For the given set of equations, which of the following choices is correct?
Ques 61 GATE 2015
For a gas phase cracking reaction A → B + C at 300°C, the Gibbs free energy of the reaction at this temperature is ΔG° = -2750 J/mol. The pressure is 1 bar. The gas phase can be assumed to be ideal. The universal gas constant R = 8.314 J/mol.K. The fractional molar conversion of A at equilibrium is
Ques 62 GATE 2015
If v,u,s and g represent respectively the molar volume, molar internal energy, molar entropy and molar Gibbs free energy, then match the entries in the left and right columns below and choose the correct option.

Ques 63 GATE 2015
An ideal gas is initially at a pressure of 0.1 MPa and a total volume of 2 m³. It is first compressed to 1 MPa by a reversible adiabatic process and then cooled at constant pressure to a final volume of 0.2 m³. The total work done (in kJ) on the gas for the entire process (up to one decimal place) is _______. Data: R = 8.314 J/mol.K; heat capacity at constant pressure (C_p) = 2.5R
Ques 64 GATE 2015
Given that molar residual Gibbs free energy, gR, and molar residual volume, vR, are related as (∂gR/∂P)T = vR, find gR at T = 27°C and P = 0.2 MPa. The gas may be assumed to follow the virial equation of state, z = 1 + BP/RT, where B = -10-4 m³/mol at the given conditions (R = 8.314 J/mol·K). The value of gR in J/mol is:
Ques 65 GATE 2015
A binary mixture of components (1) and (2) forms an azeotrope at 130°C and x1 = 0.3. The liquid phase non-ideality is described by ln γ1 = A x22 and ln γ2 = A x12, where γ1, γ2 are the activity coefficients, and x1, x2 are the liquid phase mole fractions. For both components, the fugacity coefficients are 0.9 at the azeotropic composition. Saturated vapor pressures at 130°C are P1sat = 70 bar and P2sat = 30 bar. The total pressure in bars for the above azeotropic system (up to two decimal places) is _______.

Total Unique Visitors