Chemical Engineering Gate Yearwise
Chemical Eng. Gate 2024
Chemical Eng. Gate 2023
Chemical Eng. Gate 2022
Chemical Eng. Gate 2021
Chemical Eng. Gate 2020
Chemical Eng. Gate 2019
Chemical Eng. Gate 2018
Chemical Eng. Gate 2017
Chemical Eng. Gate 2016
Chemical Eng. Gate 2015
Chemical Eng. Gate 2014
Chemical Eng. Gate 2013
Chemical Eng. Gate 2012
Chemical Eng. Gate 2011
Chemical Eng. Gate 2010
Chemical Engineering Gate 2012 Questions with Answer
Ques 53 GATE 2012
A thermocouple having a linear relationship between 0°C and 350°C shows an emf of zero and 30.5 mV, respectively at these two temperatures. If the cold junction temperature is shifted from 0°C to 30°C then the emf correction (in mV) is
Ques 54 GATE 2012
The characteristic equation for a system is s3+9s2+26s+12(2+Kc)=0. Using the Routh test, the value of Kc that will keep the system on the verge of instability is
Ques 55 GATE 2012
The block diagram of a system with a proportional controller is shown below
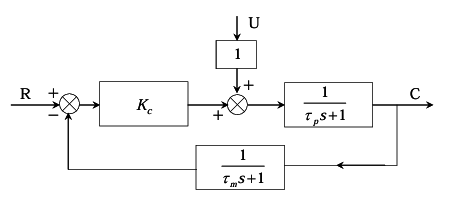
Ques 56 GATE 2012
The reaction A(liq)+B(gas)→C(liq)+D(gas) is carried out in a reactor followed by a separator as shown below
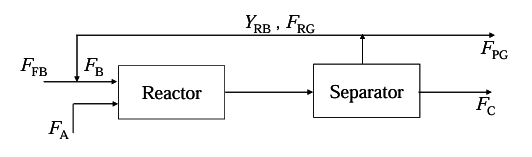
Molar flow rate of fresh B is FFB
Molar flow rate of A is FA
Molar flow rate of recycle gas is FRG
Mole fraction of B in recycle gas is YRB
Molar flow rate of purge gas is FPG
Molar flow rate of C is FC
Here, FFB=2 mol/s; FA=1 mol/s, FB/FA=5 and A is completely converted.
If YRB=0.3. the ratio of recycle gas to purge gas (FRG/FPG) is
Ques 57 GATE 2012
The reaction A(liq)+B(gas)→C(liq)+D(gas) is carried out in a reactor followed by a separator as shown below

Molar flow rate of fresh B is FFB
Molar flow rate of A is FA
Molar flow rate of recycle gas is FRG
Mole fraction of B in recycle gas is YRB
Molar flow rate of purge gas is FPG
Molar flow rate of C is FC
Here, FFB=2 mol/s; FA=1 mol/s, FB/FA=5 and A is completely converted.
If the ratio of recycle gas to purge gas (FRG/FPG) is 4 then YRB is
Ques 58 GATE 2012
In the McCabe-Thiele diagram, if the x-coordinate of the point of intersection of the q-line and the vapor-liquid equilibrium curve is greater than the x-coordinate of the feed point, then the quality of the feed is
Ques 59 GATE 2012
For which of the following combinations, does the absorption operation become gas-film controlled?
P. The solubility of gas in the liquid is very high
Q. The solubility of gas in the liquid is very low
R. The liquid-side mass transfer coefficient is much higher than the gas-side mass transfer coefficient
S. The liquid-side mass transfer coefficient is much lower than the gas-side mass transfer coefficient
Ques 60 GATE 2012
Consider the drying operation shown in the figure below for a solid loading (dry basis) of 50 kg/m2 with a constant drying rate of 5 kg/m2. The falling rate of drying is linear with moisture content.
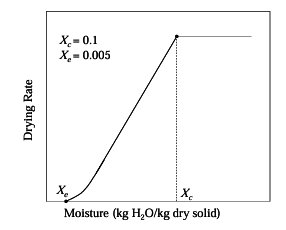
The drying time (in hrs) required to reduce an initial moisture content of 25% to a final moisture content of 2% is
Ques 61 GATE 2012
An equimolar mixture of A and B (A being more volatile) is flash distilled continuously at a feed rate of 100 kmol/h. such that the liquid product contains 40 mol % of A. If the relative volatility is 6, then the vapor product, in kmol/h is
Ques 62 GATE 2012
A counter-current extraction column is designed to remove 99% of solute C from a solution of solvent A and solute C using pure solvent B. The initial concentration of solute in the solution of A+C is 20 wt%, and the total flow of solution is 1000 kg/h. If the equilibrium relationship is Y=2X, where Y=mass of C/mass of A and X=mass of C/mass of B.
The minimum flow rate of solvent B required (in kg/h) is
Ques 63 GATE 2012
A counter-current extraction column is designed to remove 99% of solute C from a solution of solvent A
and solute C using pure solvent B. The initial concentration of solute in the solution of A + C is 20 wt %,
and the total flow of solution is 1000 kg/h. If the equilibrium relationship is Y =2X , where
Y= mass of C/mass of A and X= mass of C/mass of B.
If the flow rate of B is 2400 kg/h, then the theoretical number of stages in the column, using Kremser's equation (adjusted to the next integer) is
Ques 64 GATE 2012
In a mixing tank operating at very high Reynolds number (>104), if the diameter of the impeller is doubled (other conditions remaining constant), the power required increases by a factor of
Ques 65 GATE 2012
A batch reactor produces 1×105 kg of a product per year. The total batch time (in hours) of the reactor is k√PB, where PB is the product per batch in kg and k=1.0 h/√kg. The operating cost of the reactor is Rs. 400/h. The total annual fixed charges are Rs. 340×PB and the annual raw material cost is Rs. 2×106. The optimum size (in kg) of each batch (adjusted to the nearest integer) is

Total Unique Visitors