Metallurgical Engineering Gate Yearwise
Metallurgical Gate 2024
Metallurgical Gate 2023
Metallurgical Gate 2022
Metallurgical Gate 2021
Metallurgical Gate 2020
Metallurgical Gate 2019
Metallurgical Gate 2018
Metallurgical Gate 2017
Metallurgical Gate 2016
Metallurgical Gate 2015
Metallurgical Gate 2014
Metallurgical Gate 2013
Metallurgical Gate 2012
Metallurgical Gate 2011
Metallurgical Gate 2010
Metallurgical Gate 2009
Metallurgical Gate 2008
Metallurgical Gate 2007
Metallurgical Engineering Gate 2021 Questions with Answer
Ques 53 GATE 2021
For a zeroth order chemical reaction, which one of the following is FALSE?
Ques 54 GATE 2021
If EoNi2+/Ni = -0.25 V, the value of µoNi2+ (in J mol-1) at 298 K is: _______ (round off to nearest integer). Given: F = 96500 C mol-1
-48251 is the correct answer.
Ques 55 GATE 2021
Melting point of Cu is 1358 K and its enthalpy of melting is 13400 J mol-1. The value of free energy change (in J mol-1) for liquid to solid transformation at 1058 K is: _______ (round off to nearest integer). Assume: Cpliquid = Cpsolid
-2962 is the correct answer.
Ques 56 GATE 2021
Number of degrees of freedom for the following reacting system is:
M(s) + CO2(g) = MO(s) + CO(g)
Ques 57 GATE 2021
The condition for getting the binary phase diagram of A-B (shown below) is:
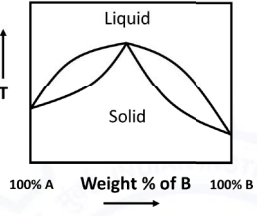
ΔHliquidmix - Enthalpy of mixing of liquid
Ques 58 GATE 2021
For the equilibrium reaction:
2Cu(s) + SO2(g) = Cu2S(s) + O2(g),
the value of ln(PO2 / PSO2) at 973 K is: _______ (round off to 2 decimal places).
Given: ΔGo at 973 K = -100 kJ
2Cu(s) + 0.5S2(g) = Cu2S(s) ΔGo at 973 K = 292 kJ
SO2(g) = 0.5S2(g) + O2(g) R = 8.314 J mol-1K-1
Assume: Cu and Cu2S are pure solids.
-23.73 is the correct answer.
Ques 59 GATE 2021
One mole of an ideal gas at 10 atm. and 300 K undergoes reversible adiabatic expansion to a pressure of one atm. The work done (in Joule) by the gas is: _______ (round off to nearest integer). Given: R = 8.314 J mol-1K-1; 1 atm. = 101325 Pa; Cp = 2.5R
2252 is the correct answer.
Ques 60 GATE 2021
The figure shows the entropy versus temperature (S-T) plot of a reversible cycle of an engine. If T1 = 200 K and T2 = 600 K, the efficiency of the engine (in percent) is: _______ (round off to 2 decimal places).
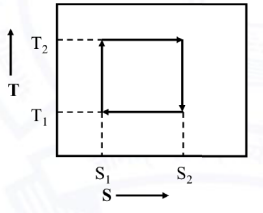
33.33 is the correct answer.
Ques 61 GATE 2021
Water flows over a plate of finite length. At x = x1 from the leading edge, the velocity of the flow is Vx = 0.5y - 0.5y3. The thickness, δ (in meter) of the boundary layer at x = x1 is: _______ (round off to 2 decimal places).
Given: V∞ is the free stream velocity.
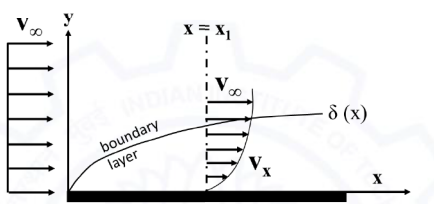
0.56 is the correct answer.
Ques 62 GATE 2021
One-dimensional steady-state temperature distribution in two adjacent refractory blocks (with thermal conductivities, k1 and k2) of unit cross-sectional area are shown below. The temperature T1 and thermal contact resistance of the interface, respectively, are:

Ques 63 GATE 2021
For a fully developed 1-D flow of a Newtonian fluid through a horizontal pipe of radius R (see figure), the axial velocity (vz) is given by:
vz = [ΔP/L](R2 - r2)/4µ
where, ΔP is the pressure difference (P1 - P2), µ is the viscosity, 'r' is the radial distance from the axis and L is the length of the tube. The shear stress exerted by the fluid on the tube wall is:
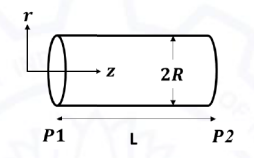
Ques 64 GATE 2021
Consider the following sentences:
(i) The number of candidates who appear for the GATE examination is staggering.
(ii) A number of candidates from my class are appearing for the GATE examination.
(iii) The number of candidates who appear for the GATE examination are staggering.
(iv) A number of candidates from my class is appearing for the GATE examination.
Which of the above sentences are grammatically CORRECT?
Ques 65 GATE 2021
The world is going through the worst pandemic in the past hundred years. The air travel industry is facing a crisis, as the resulting quarantine requirement for travelers led to weak demand. In relation to the first sentence above, what does the second sentence do?

Total Unique Visitors