Thermodynamics Mechanical previous year questions with answer
Ques 11 GATE 2024
A piston-cylinder arrangement shown in the figure has a stop located 2 m above the base. The cylinder initially contains air at 140 kPa and 350 o C and the piston is resting in equilibrium at a position which is 1 m above the stops. The system is now cooled to the ambient temperature of 25 o C. Consider air to be an ideal gas with a value of gas constant R = 0.287 k J / ( k g. K ). The absolute value of specific work done during the process is ______.(rounded off to 1 decimal place)

141.0 is the correct answer.
Ques 12 GATE 2024
A heat pump (H.P.) is driven by the work output of a heat engine (H.E.) as shown in the figure. The heat engine extracts 150 kJ of heat from the source at 1000 Κ. The heat pump absorbs heat from the ambient at 280 K and delivers heat to the room which is maintained at 300 K. Considering the combined system to be ideal, the total amount of heat delivered to the room together by the heat engine and heat pump is __________ kJ (answer in integer).

171 is the correct answer.
Ques 13 GATE 2023
Consider a mixture of two ideal gases, X and Y, with molar masses M'X = 10 kg/kmol and M'Y = 20 kg/kmol, respectively, in a container. The total pressure in the container is 100 kPa, the total volume of the container is 10 m3 and the temperature of the contents of the container is 300 K. If the mass of gas-X in the container is 2 kg, then the mass of gas-Y in the container is ____ kg.
(Rounded off to one decimal place)
Assume that the universal gas constant is 8314 J kmol-1K-1.
1.6 is the correct answer.
Ques 14 GATE 2023
A heat engine extracts heat (QH) from a thermal reservoir at a temperature of 1000 K and rejects heat (QL) to a thermal reservoir at a temperature of 100 K, while producing work (W). Which one of the combinations of [QH QI and W] given is allowed?
Ques 15 GATE 2023
Which one of the following statements is FALSE?
Ques 16 GATE 2023
Consider a fully adiabatic piston-cylinder arrangement as shown in the figure. The piston is massless and cross-sectional area of the cylinder is A. The fluid inside the cylinder is air (considered as a perfect gas), with γ being the ratio of the specific heat at constant pressure to the specific heat at constant volume for air. The piston is initially located at a position L1. The initial pressure of the air inside the cylinder is P1>>P0, where P0 is the atmospheric pressure. The stop S1 is instantaneously removed and the piston moves to the position L2, where the equilibrium pressure of air inside the cylinder is P2>>P0.
What is the work done by the piston on the atmosphere during this process?

Ques 17 GATE 2022 SET-2
Which one of the following is an intensive property of a thermodynamic system?
Ques 18 GATE 2022 SET-2
Consider 1 kg of an ideal gas at 1 bar and 300 K contained in a rigid and perfectly insulated container. The specific heat of the gas at constant volume cv is equal to 750 J∙kg-1∙K-1. A stirrer performs 225 kJ of work on the gas. Assume that the container does not participate in the thermodynamic interaction. The final pressure of the gas will be ___________ bar (in integer).
a is the correct answer.
Ques 19 GATE 2022 SET-2
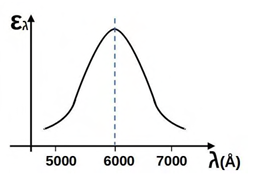
Wien’s law is stated as follows: λmT = C, where C is 2898 µm∙K and λm is the wavelength at which the emissive power of a black body is maximum for a given temperature T. The spectral hemispherical emissivity (𝜀 ) of a surface is shown in the figure below (1Å = 10-10m). The temperature at which the total hemispherical emissivity will be highest is __________ K (round off to the nearest integer).
Ques 20 GATE 2022 SET-2
In a vapour compression refrigeration cycle, the refrigerant enters the compressor in saturated vapour state at evaporator pressure, with specific enthalpy equal to 250 kJ/kg. The exit of the compressor is superheated at condenser pressure with specific enthalpy equal to 300 kJ/kg. At the condenser exit, the refrigerant is throttled to the evaporator pressure. The coefficient of performance (COP) of the cycle is 3. If the specific enthalpy of the saturated liquid at evaporator pressure is 50 kJ/kg, then the dryness fraction of the refrigerant at entry to evaporator is _________.