Chemical Engineering > GATE 2022 > Thermodynamic Relations
Consider the process in the figure. The liquid phase elementary reactions
A + B → P −r = k xA xB
A + B → S −r = k xA xB
B + A → 2P −r = k xB xA
occur in the continuous stirred tank reactor (CSTR), where x is the mole fraction of the jth component (j = A, B, P, S) in the CSTR. It is given that k = k1. All process feed, process exit and recycle streams are pure. At steady state, the net generation rate of the undesired product, S, in the CSTR is zero. As q = xA / xB is varied at constant reactor temperature, the reactor volume is adjusted to maintain a constant single-pass conversion of B. For a fixed product rate and 90% conversion of B in the reactor, the value of q that minimizes the sum of the molar flow rates of the A and S recycle streams is ________ (round off to one decimal place).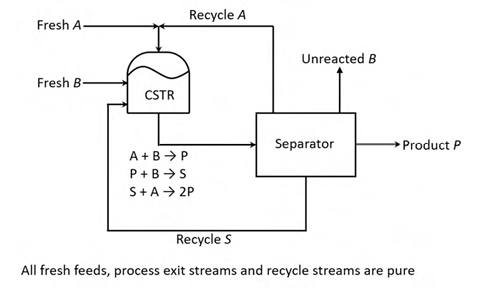
A + B → P −r = k xA xB
A + B → S −r = k xA xB
B + A → 2P −r = k xB xA
occur in the continuous stirred tank reactor (CSTR), where x is the mole fraction of the jth component (j = A, B, P, S) in the CSTR. It is given that k = k1. All process feed, process exit and recycle streams are pure. At steady state, the net generation rate of the undesired product, S, in the CSTR is zero. As q = xA / xB is varied at constant reactor temperature, the reactor volume is adjusted to maintain a constant single-pass conversion of B. For a fixed product rate and 90% conversion of B in the reactor, the value of q that minimizes the sum of the molar flow rates of the A and S recycle streams is ________ (round off to one decimal place).

Explanation
Correct : 0
Similar Questions
What is the worst-case time complexity of insertion in an AVL tree?
Which operations on a binary search tree have O(h) complexity?
Compare search complexities of sorted array vs balanced BST.