Chemical Engineering > GATE 2011 > Absorption
A gas mixture is in contact with a liquid. Component P in the gas mixture is highly soluble in the liquid. Possible concentration profiles during absorption of P are shown in the choices, where
x: mole fraction of P in bulk liquid
y: mole fraction of P in bulk gas
xi: mole fraction of P at the interface in liquid
yi: mole fraction of P at the interface in gas
y*: equilibrium gas phase mole fraction corresponding to xi
The CORRECT profile is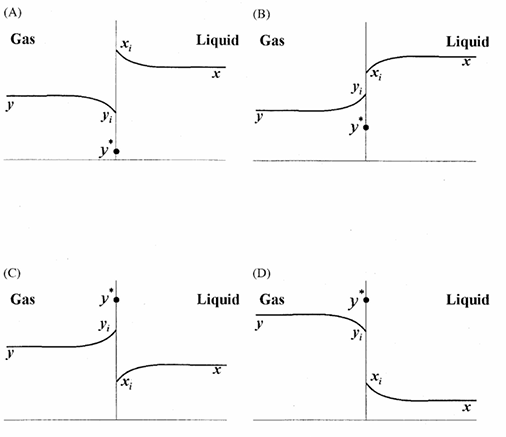
x: mole fraction of P in bulk liquid
y: mole fraction of P in bulk gas
xi: mole fraction of P at the interface in liquid
yi: mole fraction of P at the interface in gas
y*: equilibrium gas phase mole fraction corresponding to xi
The CORRECT profile is

Explanation
Correct : a
Similar Questions
What is the worst-case time complexity of insertion in an AVL tree?
Which operations on a binary search tree have O(h) complexity?
Compare search complexities of sorted array vs balanced BST.